
Although most near-surface carbonate diagenetic reactions take place in an open system, through circulation of marine or meteoric fluids, closed-system reactions can take place where permeability barriers are present. For example, a complete reorganisation (‘inversion’) of porosity can take place in carbonate rocks that are capped by impermeable sulphates (e.g. anhydrite) and salt (e.g. Hollis et al., 2017). The same interbedded carbonate-evaporite sequences can also undergo thermochemical sulphate reduction (TSR) when hydrocarbon is present, to form sour gas (H2S), in a reaction that is rate-limited by the availability of hydrocarbon (Zwahlen et al., 2019a). Reaction path modelling suggests that in such a system, ingress of CO2 will precipitate calcite, and not anhydrite, as predicted by some models informing us as to the potential behaviour of such successions during carbon sequestration (Zwahlen et al., 2019bPublications).
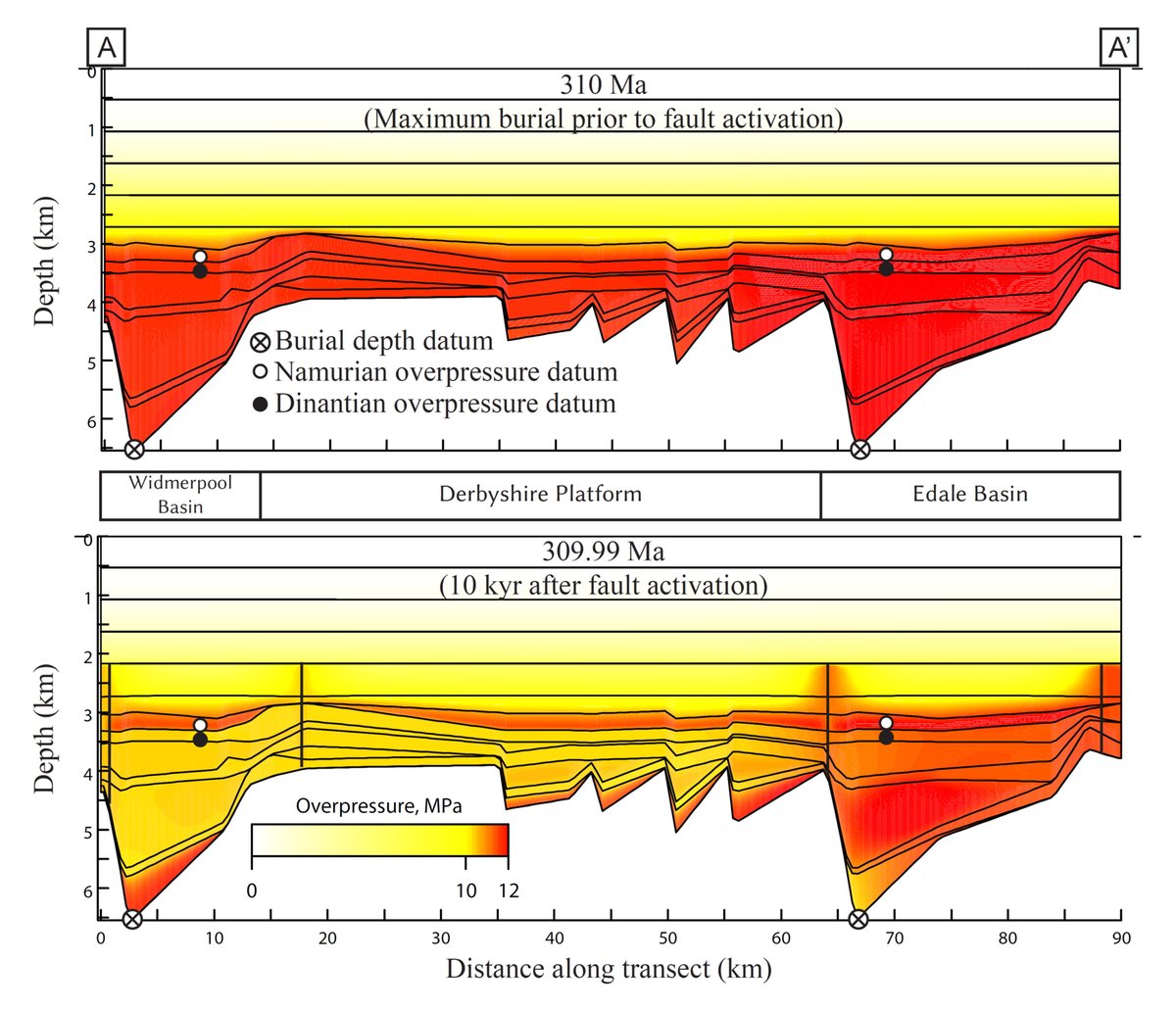
The origin of reactive fluids for diagenetic reactions within carbonate sediments remains poorly understood. Large volumes of reactive fluids can be stored in sedimentary basins, and expelled by rupture and seismic valving (e.g. Frazer et al., 2014). However, these fluids are only available for a short period of time and may not be sufficiently reactive that they can account for all the observed diagenetic changes within a sedimentary rock. New work shows that surface fluids, such as seawater, can convect along fault planes and may provide a source of diagenetic water that had previously been overlooked (e.g. Hollis et al., 2017; Hirani et al., 2018Publications).