
Skeletal oolitic limestone, Jurassic, Cotswolds, UK
Less commonly, calcium carbonate can form in lakes, from accumulation of non-marine organisms such as ostracods and microbial activity. They can also form as chemical precipitates around waterfalls (tufa) and hot springs (travertine).

Tufa deposit, Tivoli, Italy
Another common type of carbonate rock is dolostone, or dolomite (Ca.Mg.CO3). Although the World’s oceans are supersaturated with respect to Mg, dolomite does not precipitated directly from seawater. During burial of a limestone beneath overlying sediment, seawater and other warm, magnesium-rich fluids react with limestone to form dolostone (the rock) or precipitated dolomite (the mineral).
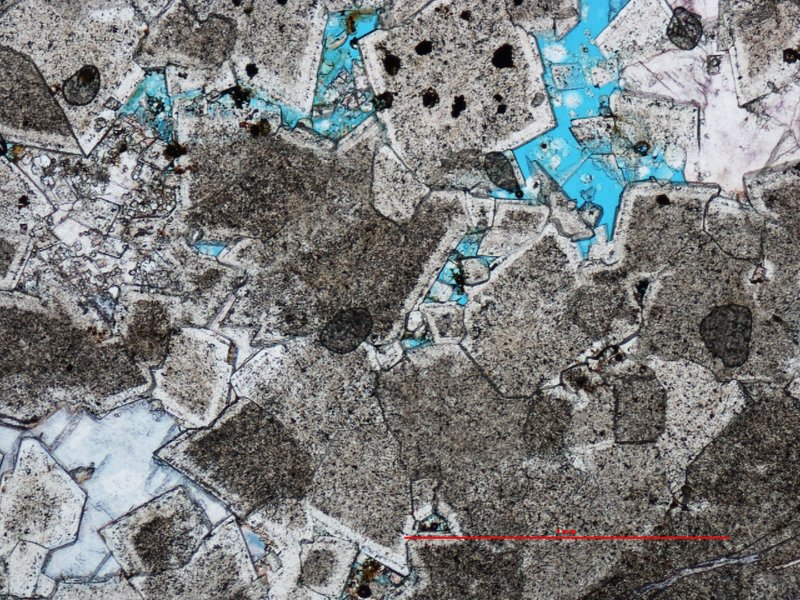
Thin section microscopic image of dolomite (scale bar = 1mm) showing coarse, rhombic-shaped crystals.
Carbonate sediments usually form in warm, shallow water away from river and delta systems, so that the water column has good light preservation. This is needed for marine organisms to photosynthesise. Sometimes the marine basins that accumulate carbonate sediment become isolated, and saline which can lead to accumulation of evaporite minerals, such as halite (NaCl) and gypsum (CaSO4.H2O). An example of this in the recent geological past occurred in the Mediterranean during the Miocene (~5 million years ago), and can be observed today along the shoreline of the UAE, Saudi Arabia and Qatar.

Halite crust with teepee structures, modern day sabkha, UAE